Mechanism of activation gating in the full-length KcsA K+ channel
By Serdar Uysal, Luis G Cuello, D Marien Cortes, Shohei Koide, Anthony Kossiakoff and Eduardo Perozo
Published in Proceedings of the National Academy of Sciences of the United States of America 108(29): 11896-9 on July 5, 2011.
PMID: 21730186. PMCID: PMC3141920. Link to Pubmed page.
Project: Dynamics of Ion Permeation and Conformational Coupling in – KcsA. Core Facility: Synthetic Antigen Binder (SAB) Generation and Crystallography.
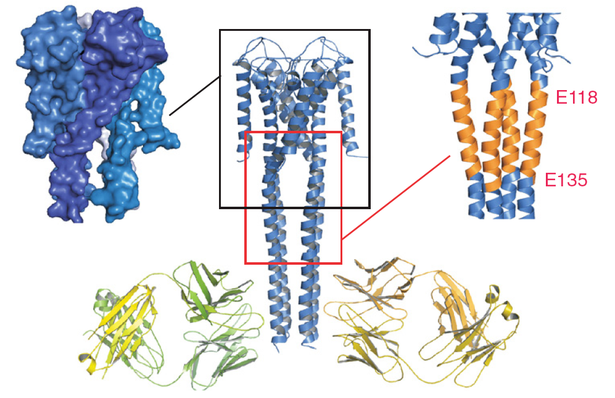
Crystal structure of FL KcsA in the open conformation. Final model of the open conformation of FL KcsA with the two Fab molecules bound with a twofold noncrystallographic symmetry to the canonical four-helix bundle portion of the cytoplasmic domain. The left inset highlights a surface representation of the open conformation showing the opening of the lower gate and the creation of associated side windows for ion flow. (Right Inset) Illustrates the expansion of the Bulge helix, comprised of residues 118–135.
Abstract
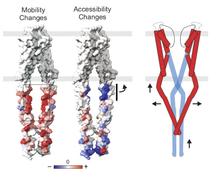
Using a constitutively active channel mutant, we solved the structure of full-length KcsA in the open conformation at 3.9 Å. The structure reveals that the activation gate expands about 20 Å, exerting a strain on the bulge helices in the C-terminal domain and generating side windows large enough to accommodate hydrated K(+) ions. Functional and spectroscopic analysis of the gating transition provides direct insight into the allosteric coupling between the activation gate and the selectivity filter. We show that the movement of the inner gate helix is transmitted to the C-terminus as a straightforward expansion, leading to an upward movement and the insertion of the top third of the bulge helix into the membrane. We suggest that by limiting the extent to which the inner gate can open, the cytoplasmic domain also modulates the level of inactivation occurring at the selectivity filter.
Publication Video
Opening of full-length KcsA: Linear interpolation of the full-length KcsA potassium channel structures in the closed and open states, depicting the process of activation gating. Residues depicted as spheres are V115.