Proof of dual-topology architecture of Fluc F- channels with monobody blockers
By Randy B. Stockbridge, Akiko Koide, Christopher Miller, and Shohei Koide.
Published in Nat Commun. 2014 Oct 7;5:5120.
PMID: 25290819. Link to publication page.
Core Facility: Synthetic Antigen Binder (SAB) Generation and Crystallography
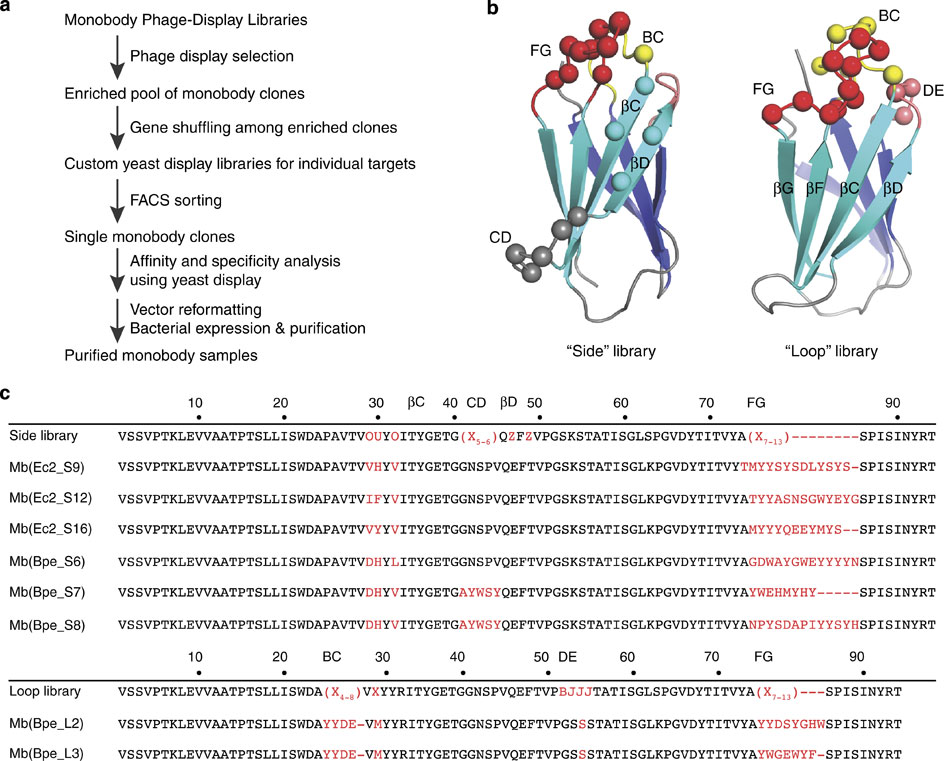
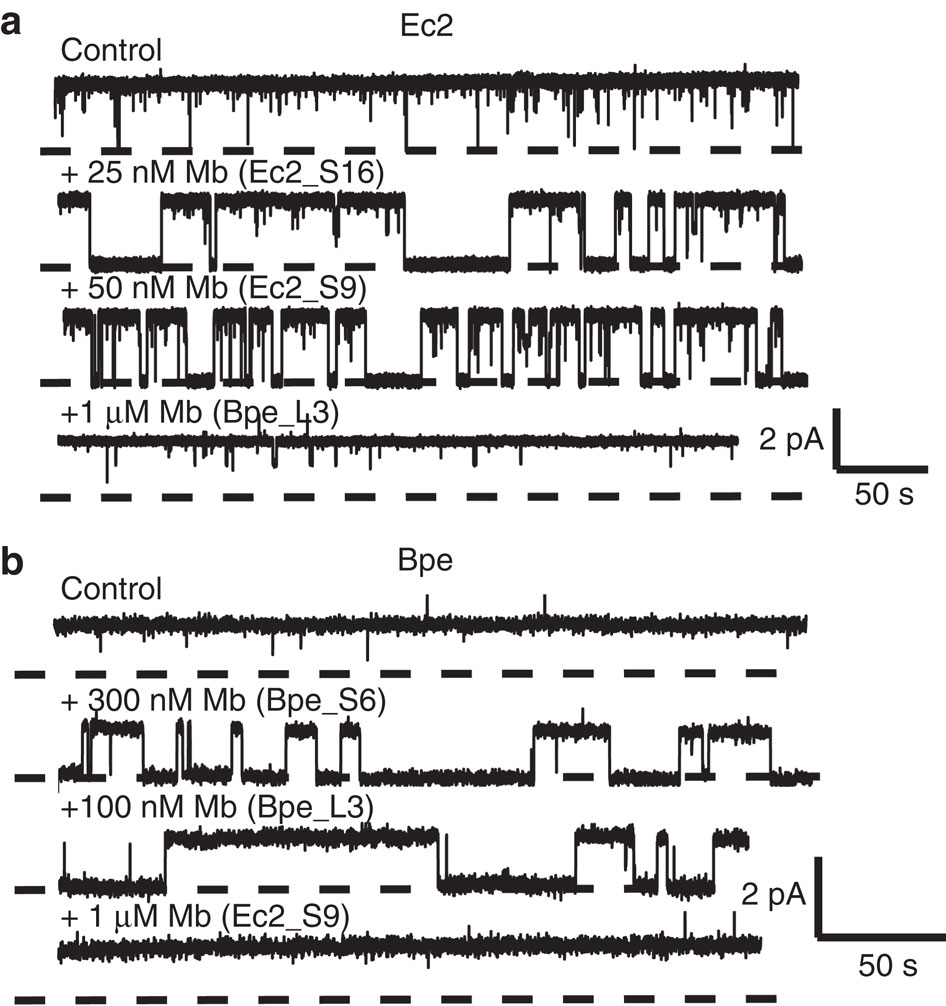
Figure 1. Selection of Fluc-directed monobodies. (a) Outline of monobody selection strategy. (b) Structure of monobody scaffold, showing residues (spheres) varied in combinatorial side and loop libraries. (c) Sequences of monobodies (variable regions in red) selected against Fluc homologues Ec2 and Bpe from side and loop libraries as indicated by ‘S’ or ‘L’ designators in monobody labels. All eight monobodies bind to their targets with submicromolar dissociation constants, and all except Mb(Bpe_S8) block Fluc channels in electrical recording assays. Upper sequence of each library indicates tailored variation as follows: ‘X’ denotes a mixture of 30% Tyr, 15% Ser, 10% Gly, 5% Phe, 5% Trp and 2.5% each of all the other amino acids except for Cys; ‘B’, a mixture of Gly, Ser and Tyr; ‘J’, a mixture of Ser and Tyr; ‘O’, a mixture of Asn, Asp, His, Ile, Leu, Phe, Tyr and Val; ‘U’, a mixture of His, Leu, Phe and Tyr; ‘Z’, a mixture of Ala, Glu, Lys and Thr, as previously reported.
Abstract
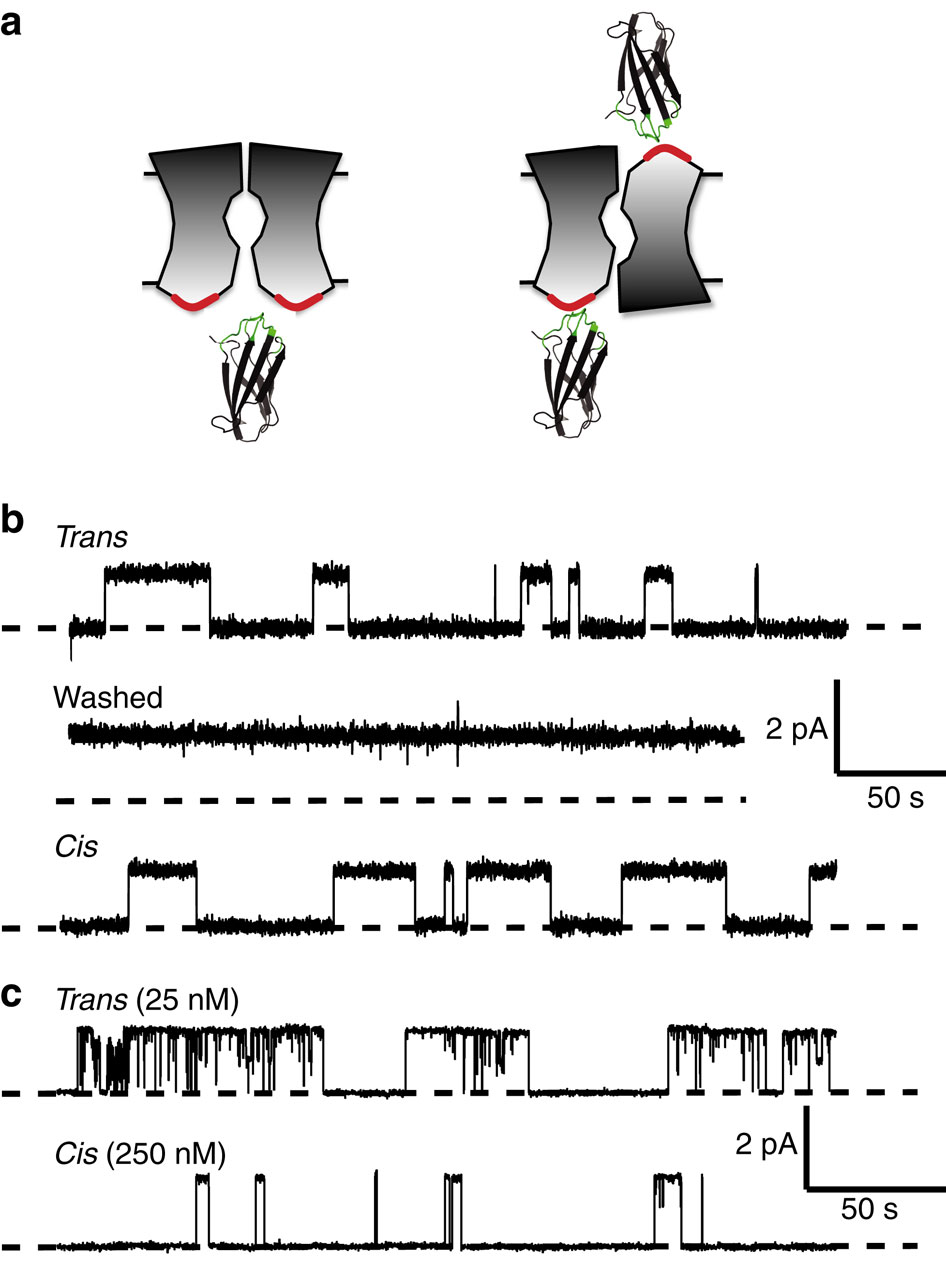
Fluc-type F(-) channels–used by microorganisms for resisting fluoride toxicity–are unusual in their quaternary architecture: they are thought to associate as dimers with the two subunits in antiparallel transmembrane orientation. Here, we subject this unusual structural feature to a direct test. Single purified Fluc channels recorded in planar lipid bilayers are constitutively open, with rare, short-lived closings. Using combinatorial libraries, we generated synthetic binding proteins, ‘monobodies,’ that specifically bind to Fluc homologues with nanomolar affinity. Reversible binding of monobodies to two different Fluc channel homologues is seen in single-channel recordings as long-lived nonconducting events that follow bimolecular kinetics. By applying monobodies sequentially to the two sides of the bilayer in a double-sided perfusion manoeuvre, we show that Fluc channels present monobody-binding epitopes to both sides of the membrane. The result establishes that Fluc subunits are arranged in dimeric antiparallel orientation.