Mechanistic picture for conformational transition of a membrane transporter at atomic resolution
By Mahmoud Moradi and Emad Tajkhorshid.
Published in Proceedings of the National Academy of Sciences of the United States of America 110(47): 18916-21 on November 19, 2013. PMID: 24191018. PMCID: PMC3839739. Link to publication page.
Project: Structural Dynamics of ABC Transporter. Core Facility: Computational Modeling.
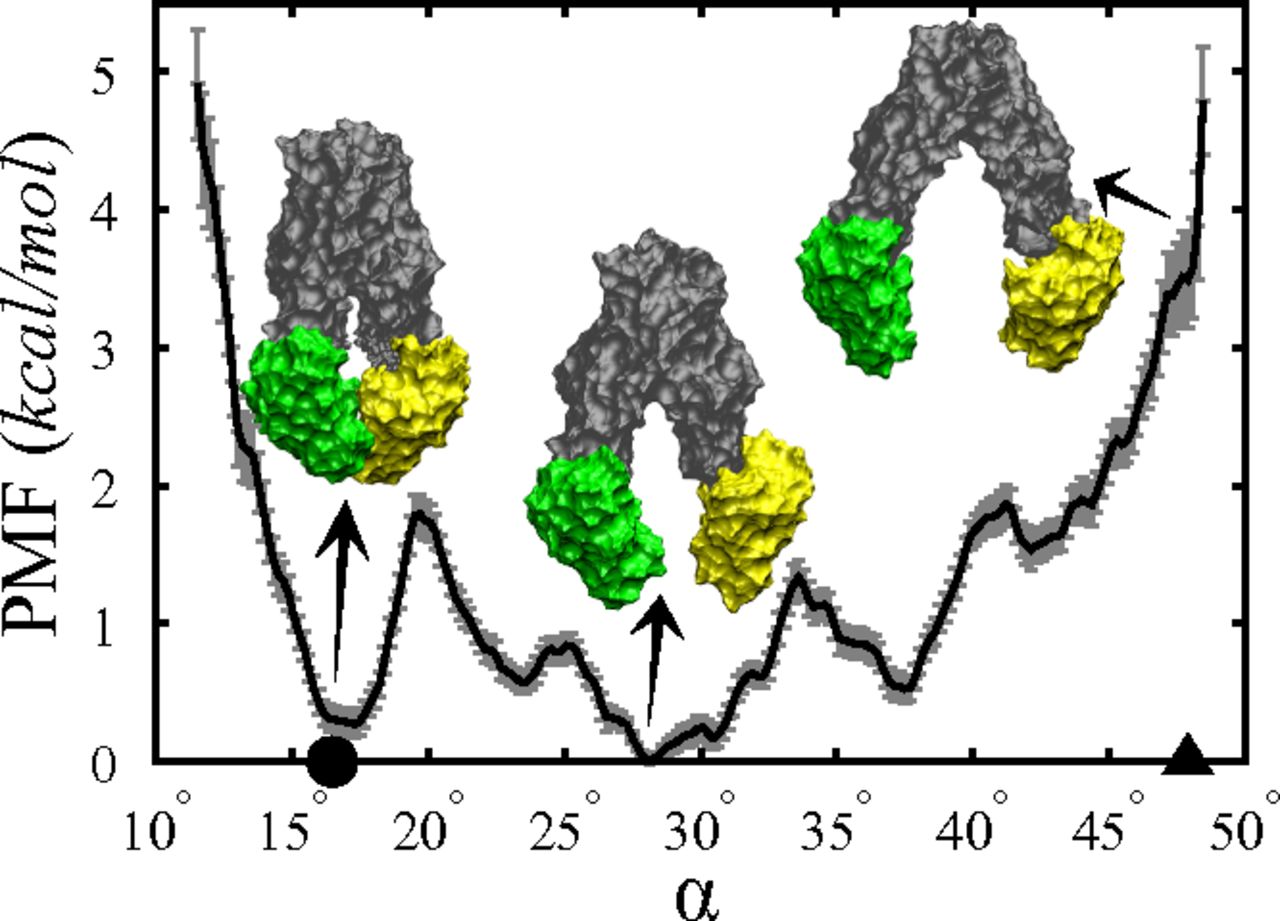
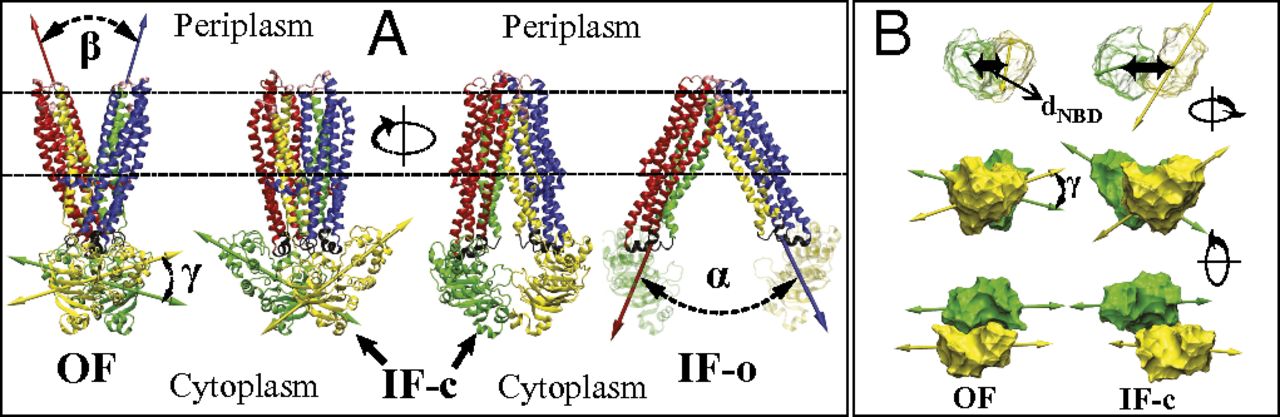
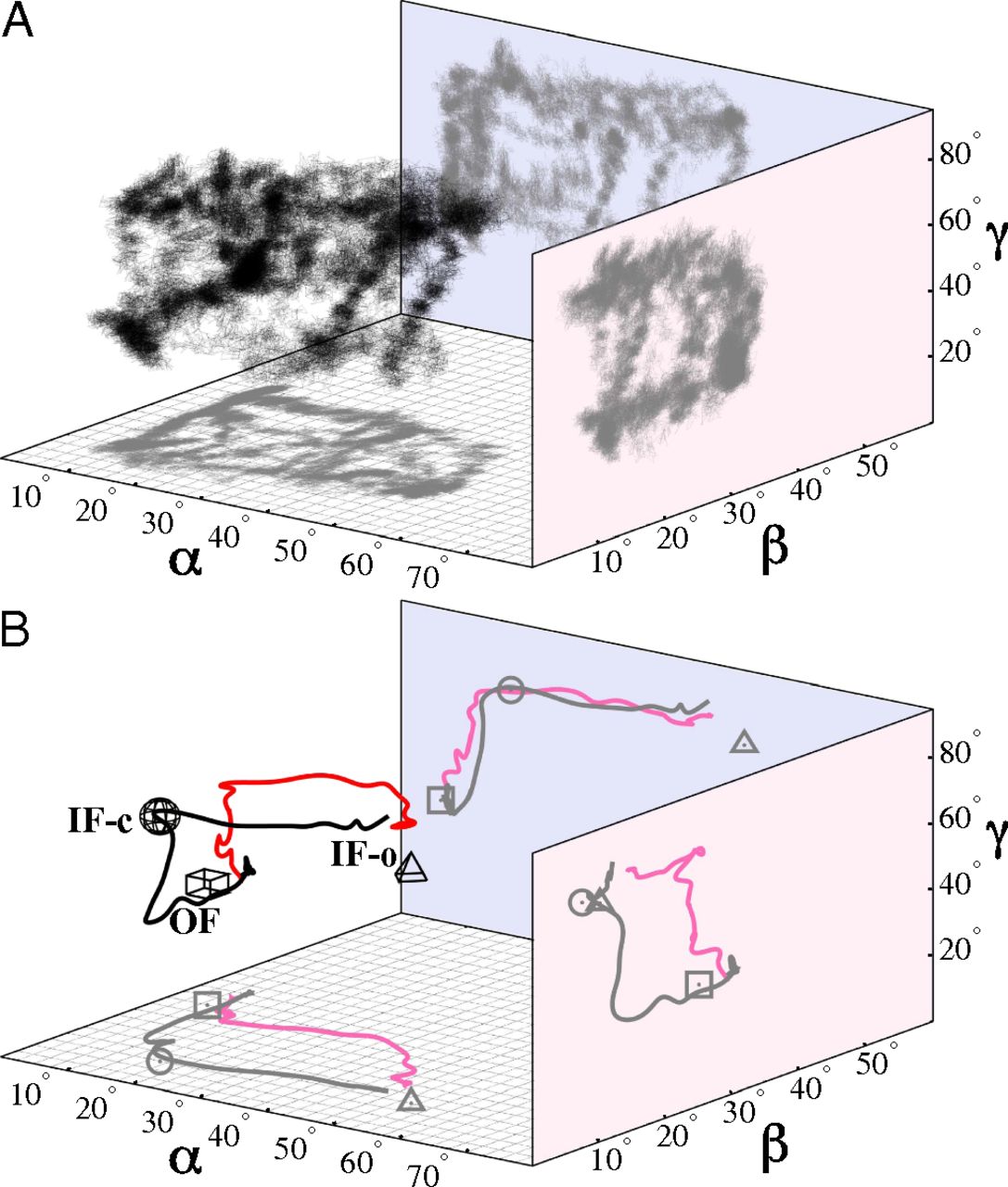
Figure 5. PMF of apo MsbA along α in the IF state. The PMF is obtained from BEUS MD simulations, and the error bars are estimated using a bootstrapping algorithm (SI Appendix). The MsbA conformations shown (in surface representation) represent the IF-c and IF-o crystal structures (left and right, respectively), as well as an IF conformation (center) that resembles the P-gp crystal structures (8⇓–10). The values of α associated with the IF-c and IF-o crystal structures are marked by a circle and a triangle, respectively.
Significance
Membrane transporters rely on large-scale conformational changes for their function. Despite considerable effort, however, details of such structural transitions are largely unknown, leaving many fundamental questions regarding the transport mechanism in this important family of membrane proteins unanswered. Here, we present a nonequilibrium approach to characterize the conformational transition of MsbA, a member of the ATP-binding cassette exporter family, which is involved in transport of diverse substrates across the membrane. The design of the study is based on complex, system-specific reaction coordinates and protocols resulting in an unprecedented level of detail on the nature of conformational coupling and the mechanism of transport. The presented approach opens opportunities for investigating large-scale conformational changes of other membrane transporters.
Abstract
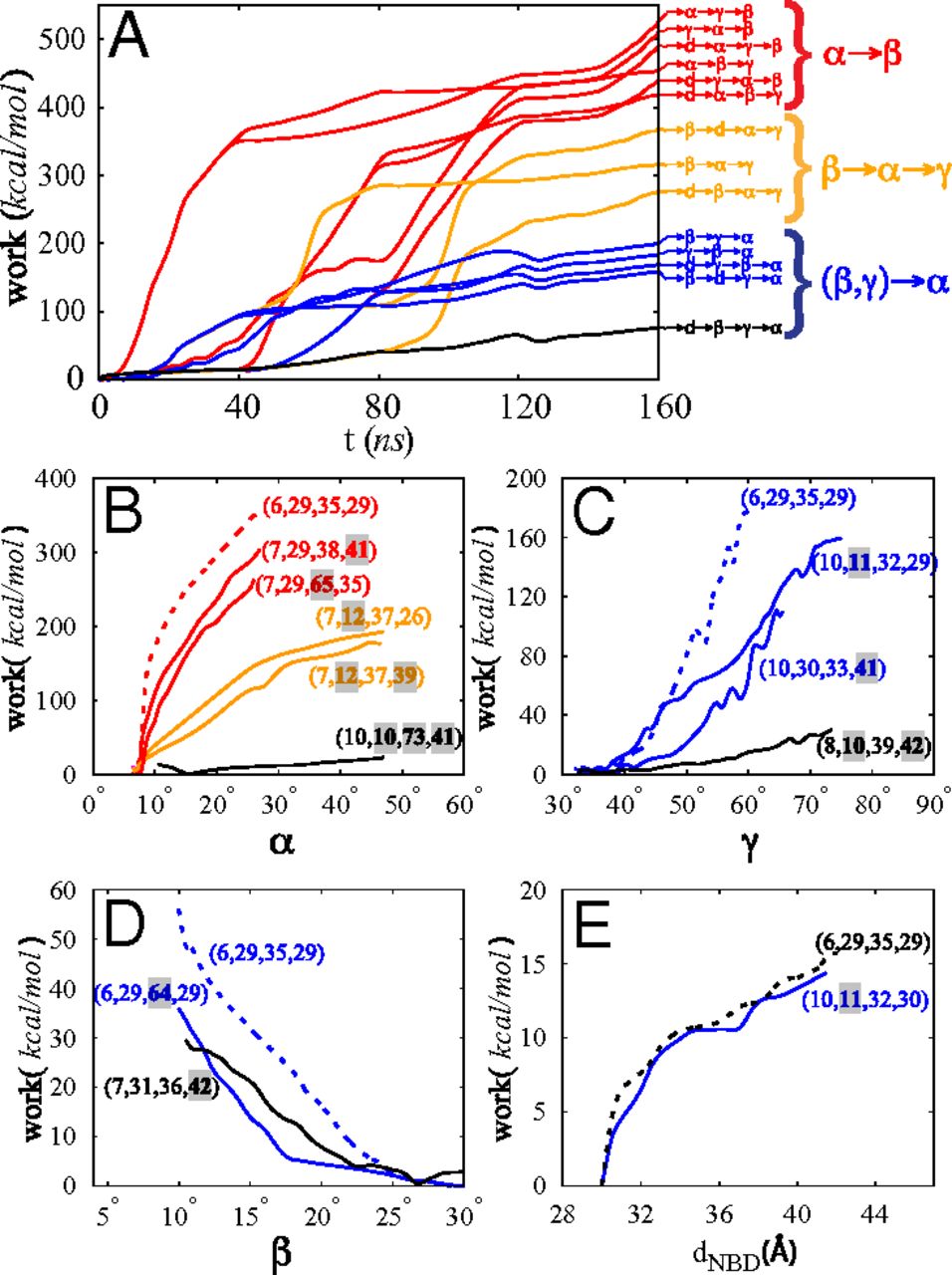
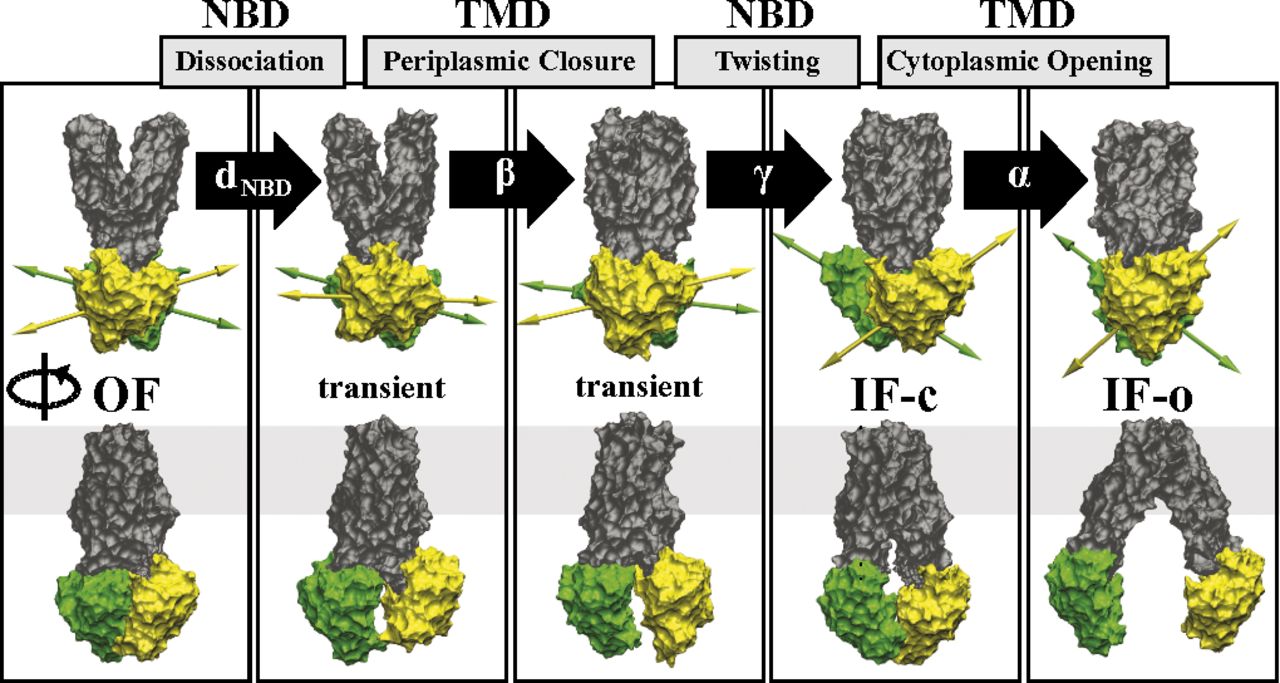
During their transport cycle, ATP-binding cassette (ABC) transporters undergo large-scale conformational changes between inward- and outward-facing states. Using an approach based on designing system-specific reaction coordinates and using nonequilibrium work relations, we have performed extensive all-atom molecular dynamics simulations in the presence of explicit membrane/solvent to sample a large number of mechanistically distinct pathways for the conformational transition of MsbA, a bacterial ABC exporter whose structure has been solved in multiple functional states. The computational approach developed here is based on (i) extensive exploration of system-specific biasing protocols (e.g., using collective variables designed based on available low-resolution crystal structures) and (ii) using nonequilibrium work relations for comparing the relevance of the transition pathways. The most relevant transition pathway identified using this approach involves several distinct stages reflecting the complex nature of the structural changes associated with the function of the protein. The opening of the cytoplasmic gate during the outward- to inward-facing transition of apo MsbA is found to be disfavored when the periplasmic gate is open and facilitated by a twisting motion of the nucleotide-binding domains that involves a dramatic change in their relative orientation. These results highlight the cooperativity between the transmembrane and the nucleotide-binding domains in the conformational transition of ABC exporters. The approach introduced here provides a framework to study large-scale conformational changes of other membrane transporters whose computational investigation at an atomic resolution may not be currently feasible using conventional methods.