Target-binding proteins based on the 10th human fibronectin type III domain (10Fn3)
By Shohei Koide, Akiko Koide, and Daša Lipovšek.
Published in Methods in Enzymology 503: 135-56 on February 2012.
PMID: 22230568. Link to Pubmed page.
Core Facility: Synthetic Antigen Binder (SAB) Generation and Crystallography
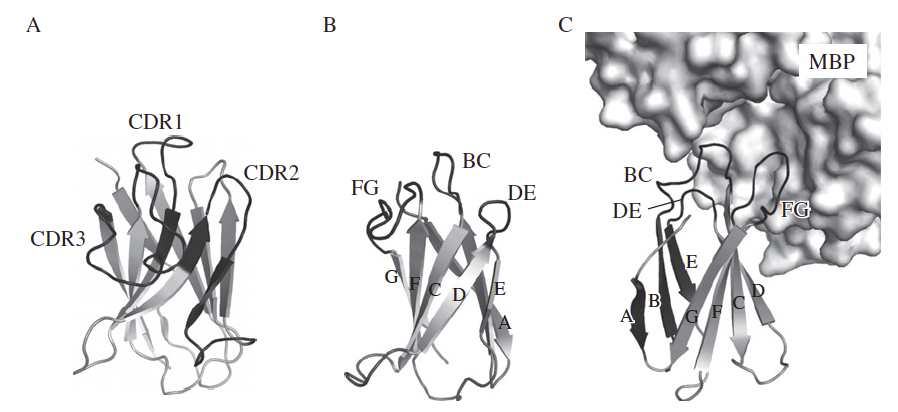
Schematic drawing of the crystal structures of the antigen-binding domain of the camelid heavy-chain antibody (VHH; PDB ID, 1ZVY; A), the 10Fn3 scaffold (PDB ID, 1FNF; B), and a 10Fn3-based binding protein (PDB ID, 3CSB; C). (A) The three complementarity-determining regions (CDRs) are shown in black and also labeled. (B and C) The b-strands of the 10Fn3 scaffold are labeled with A-G and the three loops used for constructing a new binding site are shown in black and also labeled. (C) A portion of the target molecule, maltose-binding protein (MBP), is shown as a surface model.
Abstract
We describe concepts and methods for generating a family of engineered target-binding proteins designed on the scaffold of the 10th human fibronectin type III domain (10Fn3), an extremely stable, single-domain protein with an immunoglobulin-like fold but lacking disulfide bonds. Large libraries of possible target-binding proteins can be constructed on the 10Fn3 scaffold by diversifying the sequence and length of its surface loops, which are structurally analogous to antibody complementarity-determining regions. Target-binding proteins with high affinity and specificity are selected from 10Fn3-based libraries using in vitro evolution technologies such as phage display, mRNA display, or yeast-surface display. 10Fn3-based target-binding proteins have binding properties comparable to those of antibodies, but they are smaller, simpler in architecture, and more user-friendly; as a consequence, these proteins are excellent building blocks for the construction of multidomain, multifunctional chains. The ease of engineering and robust properties of 10Fn3-based target-binding proteins have been validated by multiple independent academic and industrial groups. In addition to performing well as specific in vitro detection reagents and research tools, 10Fn3-based binding proteins are being developed as therapeutics, with the most advanced candidate currently in Phase II clinical trials.


