Welcome to the MPSDC Gateway!
Membrane proteins play an essential role in controlling the movement of material and information in and out of the cell, in determining the flow and use of energy, as well as in triggering the initiation of numerous signaling pathways. To fulfill these roles, conformational and interaction dynamics exert a dominant influence on their functional behavior, for it is the interplay between structure and dynamics what ultimately defines their function.
The Membrane Protein Structural Dynamics Consortium (MPSDC) has been designed as a highly interactive, tightly integrated and multidisciplinary effort focused on elucidating the relationship between structure, dynamics and function in a variety of membrane proteins. This website serves as a gateway both to the Consortium's activities and resources, and to the scientific field at large.
- July 3, 2019: 2019 Biological CryoEM workshop video and photos released
- March 22, 2019: 2019 Workshop on Advances and Challenges of Biological CryoEM
- March 9, 2018: Third Coast Workshop on Biological Cryo-EM announced for May 19, 2018
- November 8, 2017: Final Program for III Frontiers in Membrane Protein Structural Dynamics Announced
- June 23, 2017: Announcing Frontiers in Membrane Protein Structural Dynamics 2017
- February 15, 2017: Emad Tajkhorshid and co-PIs at UIUC recipients of NIH High-Risk, High-Reward Research View more entries »
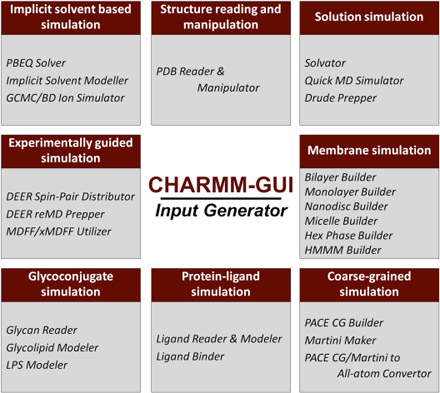
- CHARMM-GUI 10 years for biomolecular modeling and simulation (Published on June 5, 2017)
- The role of transmembrane segment 5 (TM5) in Na2 release and the conformational transition of neurotransmitter:sodium symporters toward the inward-open state (Published on May 5, 2017)
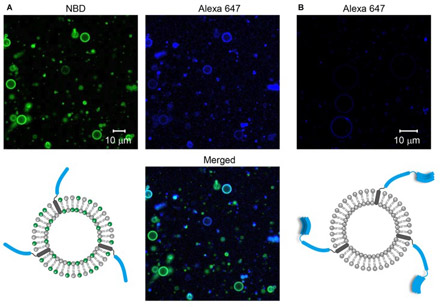
- Visualization of SNARE-Mediated Hemifusion between Giant Unilamellar Vesicles Arrested by Myricetin (Published on March 31, 2017)
- Energy transduction and alternating access of the mammalian ABC transporter P-glycoprotein (Published on March 30, 2017)
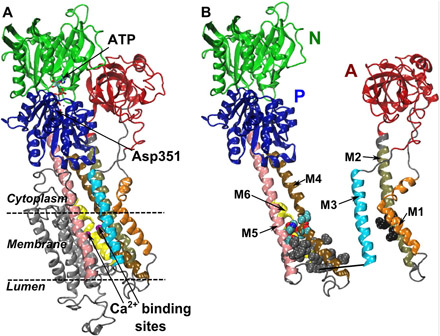
- Conformational Transitions and Alternating-Access Mechanism in the Sarcoplasmic Reticulum Calcium Pump (Published on January 16, 2017) View more entries »
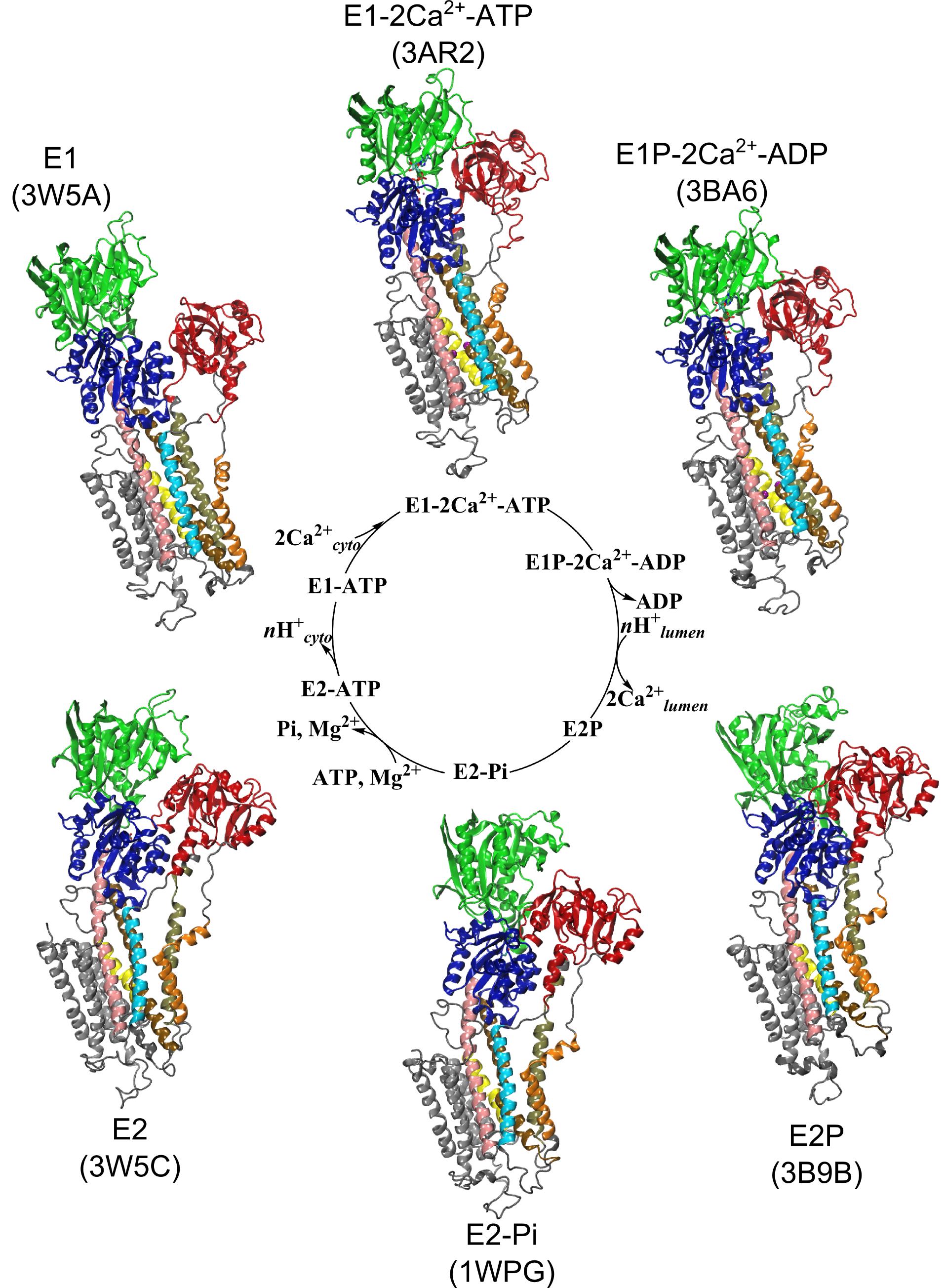 Ion pumps are integral membrane proteins responsible for transporting ions against concentration gradients across biological membranes. Computational methods can be used to help supplement missing information about these pumps by providing atomic models for the transient intermediates along the transport cycle. Our goal with the present effort was to elucidate the details of the alternating access mechanism in SERCA by simulating the large-scale conformational transitions between the experimentally resolved stable sates.
Ion pumps are integral membrane proteins responsible for transporting ions against concentration gradients across biological membranes. Computational methods can be used to help supplement missing information about these pumps by providing atomic models for the transient intermediates along the transport cycle. Our goal with the present effort was to elucidate the details of the alternating access mechanism in SERCA by simulating the large-scale conformational transitions between the experimentally resolved stable sates. Learn more »
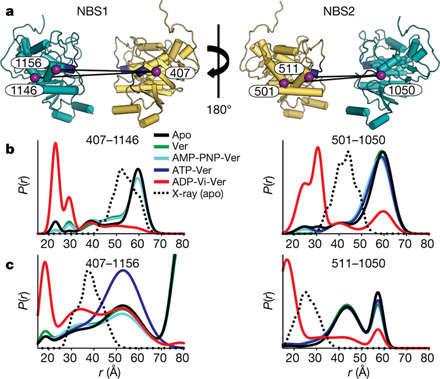
The conceptual design, scope and integration of the Transport Cycle in Neurotransmitter Uptake Project exemplifies the consortium approach to discovery of mechanistic principles of secondary active transport. Read more »
Watch the video »