The selectivity of the Na(+)/K(+)-pump is controlled by binding site protonation and self-correcting occlusion
By Huan Rui, Pablo Artigas, and Benoît Roux.
Published in Elife 2016 Aug 4;5. pii: e16616. PMID: 27490484. PMCID: PMC5026471. Link to Pubmed page.
Core Facility: Computational Modeling. Project: Conformational Transitions in P-class ATPases
Abstract
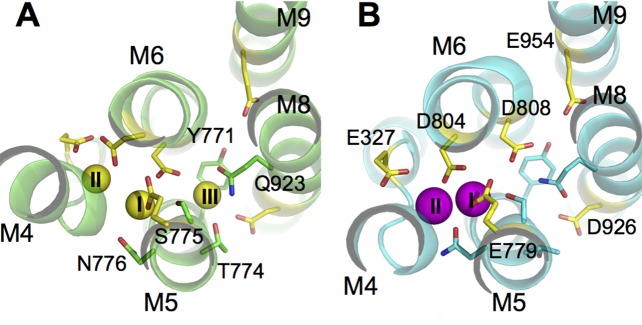
The Na(+)/K(+)-pump maintains the physiological K(+) and Na(+) electrochemical gradients across the cell membrane. It operates via an ‘alternating-access’ mechanism, making iterative transitions between inward-facing (E1) and outward-facing (E2) conformations. Although the general features of the transport cycle are known, the detailed physicochemical factors governing the binding site selectivity remain mysterious. Free energy molecular dynamics simulations show that the ion binding sites switch their binding specificity in E1 and E2. This is accompanied by small structural arrangements and changes in protonation states of the coordinating residues. Additional computations on structural models of the intermediate states along the conformational transition pathway reveal that the free energy barrier toward the occlusion step is considerably increased when the wrong type of ion is loaded into the binding pocket, prohibiting the pump cycle from proceeding forward. This self-correcting mechanism strengthens the overall transport selectivity and protects the stoichiometry of the pump cycle.


