Conformational cycle and ion-coupling mechanism of the Na+/hydantoin transporter Mhp1
By Kelli Kazmier, Shruti Sharma, Shahidul M Islam, Benoît Roux, and Hassane Mchaourab.
Published in Proc Natl Acad Sci U S A. 2014 Oct 14;111(41):14752-7. PMID: 25267652. PMCID: PMC4205665. Link to publication page.
Project: The Transport Cycle in Neurotransmitter Uptake Systems. Core Facility: Computational Modeling.
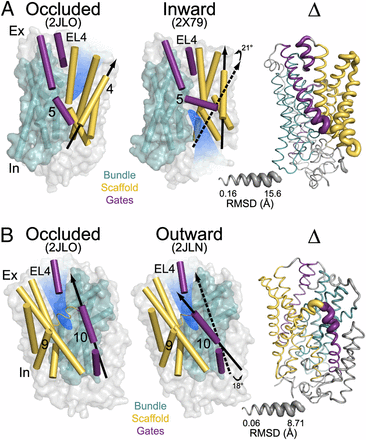
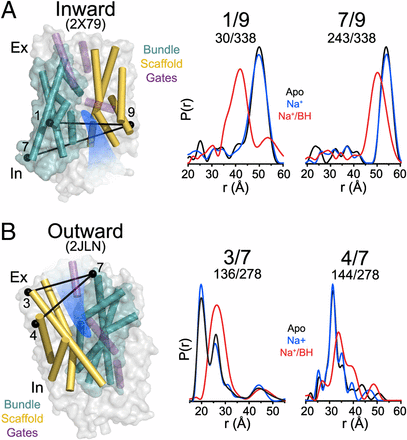
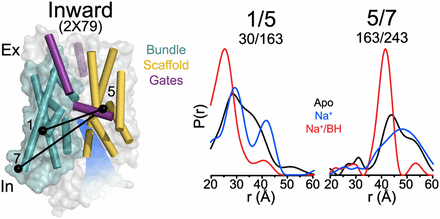
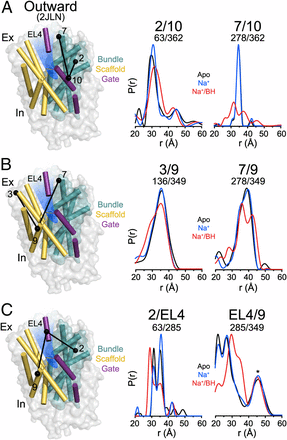
Figure 1. (A) Comparison of outward-facing (2JL0) and inward-facing crystal structures (2X79) of Mhp1 highlighting the inferred movement of the scaffold (yellow) relative to the bundle (cyan). The rmsd between the two structures is displayed by the ribbon thickness (scale in gray) on the 2JLO backbone (Δ). The change in orientation of the scaffold between the two structures is shown by the arrows. The approximate location of the solvent accessible vestibule is indicated in blue. (B) Comparison of outward-facing conformation (2JLO) and outward-facing/occluded conformation (2JLN) highlighting the inferred movement of TM10.
Significance
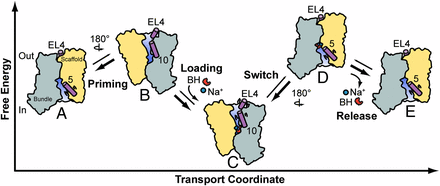
Na+-coupled symporters use the cellular Na+ gradient to power transport of physiologically important molecules across the lipid membrane. However, the mechanism by which binding and dissociation of Na+ drive transport remains undefined. This work investigated the Na+/hydantoin transporter Mhp1, a member of the LeuT-fold class of transporters, to describe the conformations sampled during its transport cycle and elucidate the ligand-induced shifts in its conformational equilibrium. The results of this study suggest that Mhp1 isomerization between inward- and outward-facing conformations are Na+-independent and that coupling to the Na+ gradient occurs through modulation of substrate affinity by Na+ coordination. A previously unidentified model of Mhp1 transport defined by ligand-independent equilibrium fluctuations emerges from this work, offering a new perspective on Na+-coupled symport in the LeuT-fold.
Abstract
Ion-dependent transporters of the LeuT-fold couple the uptake of physiologically essential molecules to transmembrane ion gradients. Defined by a conserved 5-helix inverted repeat that encodes common principles of ion and substrate binding, the LeuT-fold has been captured in outward-facing, occluded, and inward-facing conformations. However, fundamental questions relating to the structural basis of alternating access and coupling to ion gradients remain unanswered. Here, we used distance measurements between pairs of spin labels to define the conformational cycle of the Na+-coupled hydantoin symporter Mhp1 from Microbacterium liquefaciens. Our results reveal that the inward-facing and outward-facing Mhp1 crystal structures represent sampled intermediate states in solution. Here, we provide a mechanistic context for these structures, mapping them into a model of transport based on ion- and substrate-dependent conformational equilibria. In contrast to the Na+/leucine transporter LeuT, our results suggest that Na+ binding at the conserved second Na+ binding site does not change the energetics of the inward- and outward-facing conformations of Mhp1. Comparative analysis of ligand-dependent alternating access in LeuT and Mhp1 lead us to propose that different coupling schemes to ion gradients may define distinct conformational mechanisms within the LeuT-fold class.