Importance of lipid-pore loop interface for potassium channel structure and function
By Elwin A. W. van der Cruijsen, Deepak Nand, Markus Weingarth, Alexander Prokofyev, Sönke Hornig, Abhishek Arun Cukkemane, Alexandre M. J. J. Bonvin, Stefan Becker, Raymond E. Hulse, Eduardo Perozo, Olaf Pongs, and Marc Baldus.
Published in Proceedings of the National Academy of Sciences of the USA, 110(32):13008-13 on August 6, 2013. PMID: 23882077. PMCID: PMC3740848. Link to publication page.
Project: Dynamics of Ion Permeation and Conformational Coupling in – KcsA
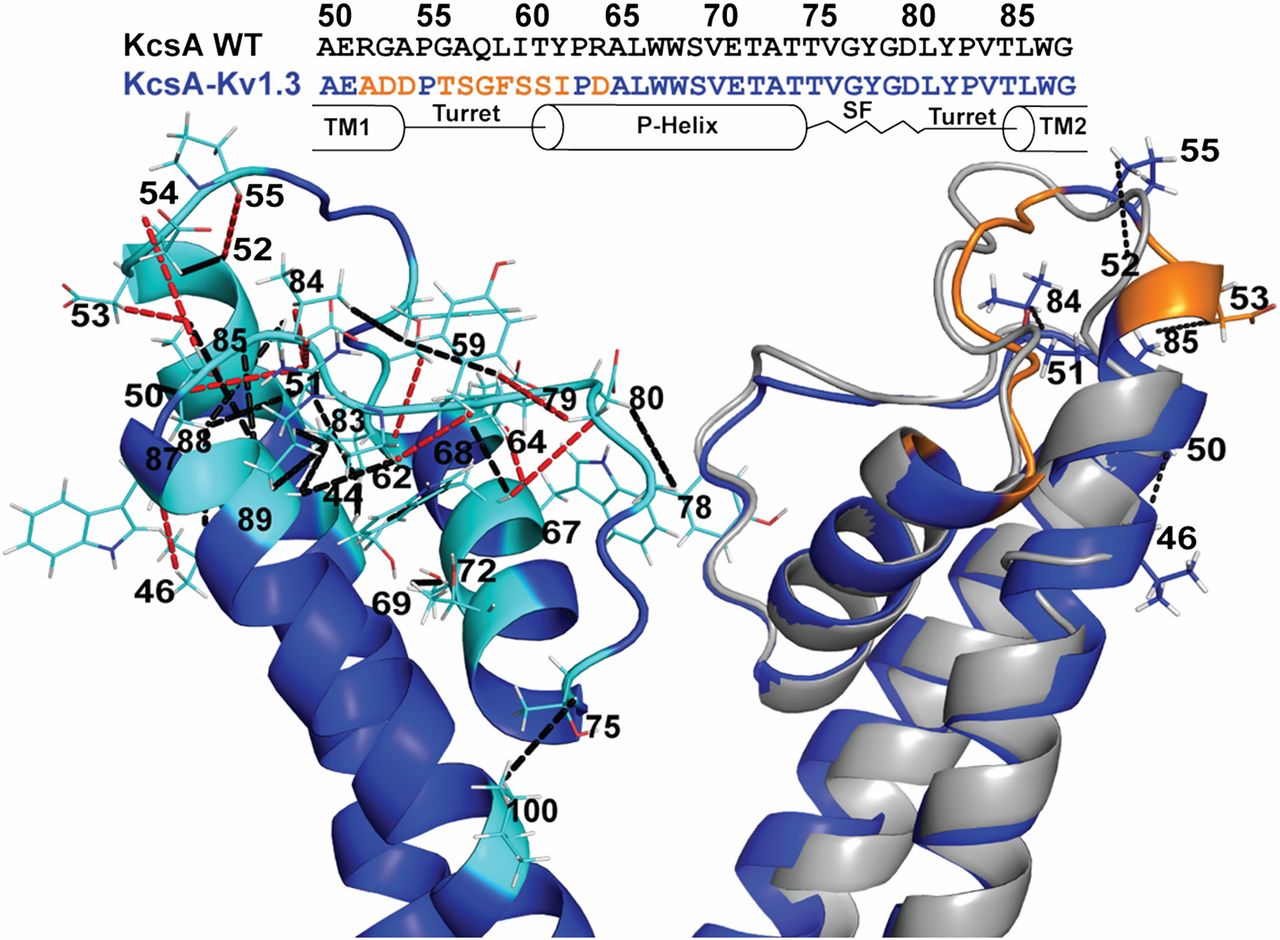
Figure 1. SsNMR analysis of membrane-embedded Chim in the closed conductive state. (Left) Cartoon representation of the 3D ssNMR model of the closed state (residues Chim 22–115). Resolved CHHC restraints (highlighted in cyan) identified from the CHHC spectrum (250 µs and 500 µs 1H-1H mixing) that are unambiguous based on the KcsA crystal structure and the available chemical shifts are indicated by black dashed lines. Additional correlations unique for the ssNMR structure of Chim are shown by red dashed lines. (Right) Superposition of the Chim structural model (blue) and the KcsA crystal structure (gray) with three resolved ssNMR restraints confirming the extended α-helical turn for he chimeric channel along with their amino acid sequence comparison highlighting (orange) the 11 mutations distinguishing the turret region.
Abstract
Potassium (i.e., K+) channels allow for the controlled and selective passage of potassium ions across the plasma membrane via a conserved pore domain. In voltage-gated K+ channels, gating is the result of the coordinated action of two coupled gates: an activation gate at the intracellular entrance of the pore and an inactivation gate at the selectivity filter. By using solid-state NMR structural studies, in combination with electrophysiological experiments and molecular dynamics simulations, we show that the turret region connecting the outer transmembrane helix (transmembrane helix 1) and the pore helix behind the selectivity filter contributes to K+ channel inactivation and exhibits a remarkable structural plasticity that correlates to K+ channel inactivation. The transmembrane helix 1 unwinds when the K+ channel enters the inactivated state and rewinds during the transition to the closed state. In addition to well-characterized changes at the K+ ion coordination sites, this process is accompanied by conformational changes within the turret region and the pore helix. Further spectroscopic and computational results show that the same channel domain is critically involved in establishing functional contacts between pore domain and the cellular membrane. Taken together, our results suggest that the interaction between the K+ channel turret region and the lipid bilayer exerts an important influence on the selective passage of potassium ions via the K+ channel pore.

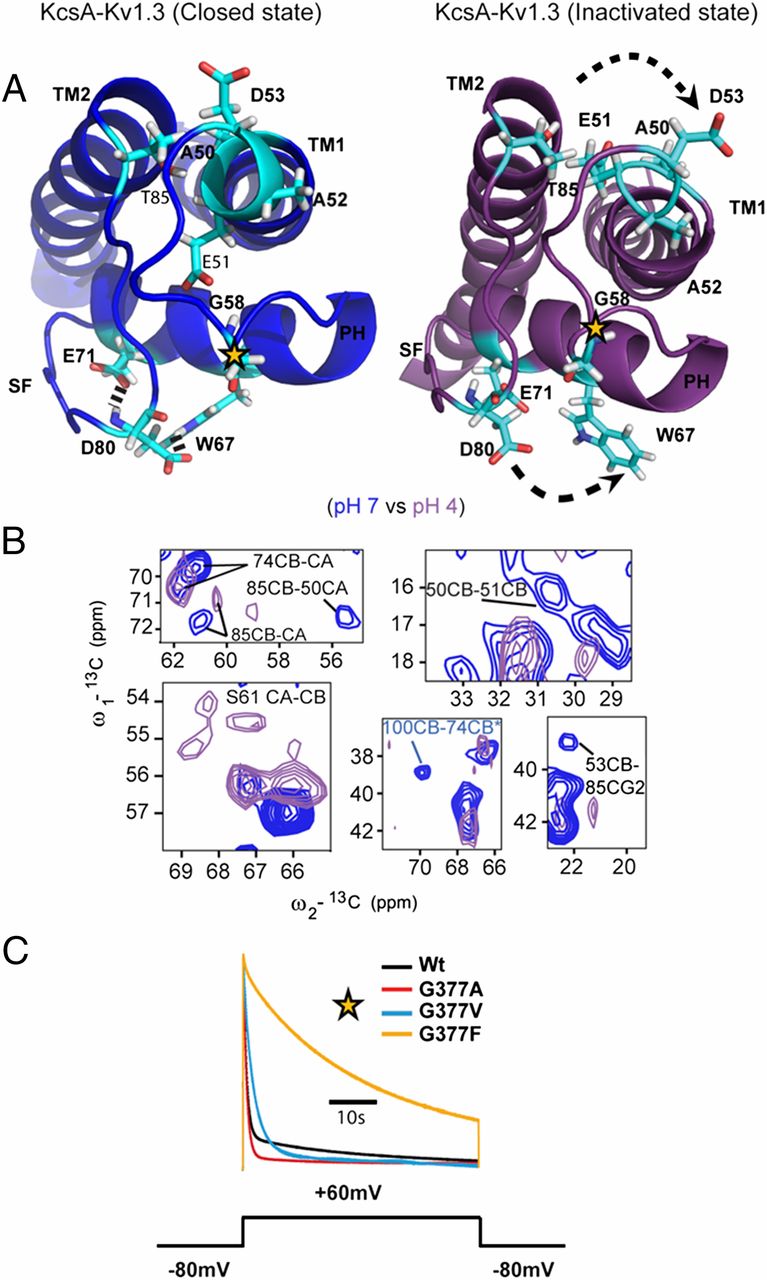
![Figure 3. (A) ssNMR-based structural model of membrane-embedded KcsA (WT) in the closed conductive state. (Left) Cartoon representation of the 3D ssNMR model of the closed state of KcsA (residues WT 22–115). Resolved CHHC restraints (highlighted in cyan) identified from the CHHC spectrum (250 µs 1H-1H mixing) that are unambiguous based on the ssNMR assignments. (Right) Superposition of the KcsA structural model (red), KcsA-Kv.1.3 (blue), and KcsA crystal structure (gray). (B) WTom Glu71 mutants change the conformation of the pore-loop of KcsA. (Left) Cartoon representation of KcsA with the mutation site E71 (purple), with residues exhibiting ssNMR shifts (blue) or peak doubling in the two mutants. (Right) (13C,13C) Correlation spectra obtained for WT (red), WTom E71Q, and WTom E71A (green and black, respectively), at pH 7.4, 50 mM [K+] reconstituted in asolectin.](/site-media/images/publications/2013/perozo-baldus-2013-figure3.jpg)
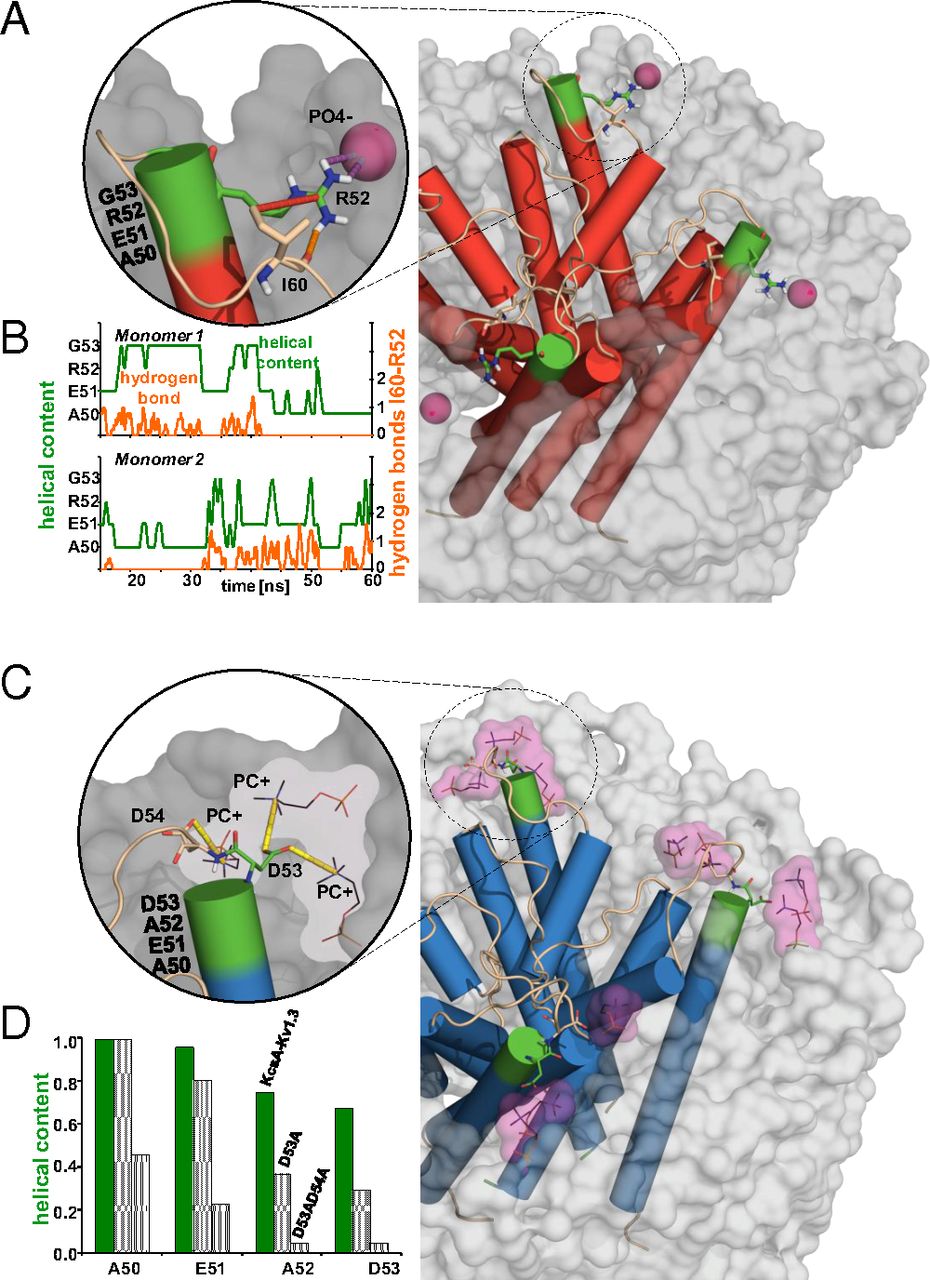
![Figure 5. Role of interfacial and turret residues in K+ channel gating and channel stabilization. (A) Superposition of the inactivated state of Chim channel (gray) and the X-ray structure of the cyclic nucleotide-regulated K+ channel MlotiK1 [light blue (45); PDB ID code 3BEH] obtained by aligning the S5–S6 subunits. Residues highlighted in red (Chim-Ala50, Glu51, Asp64, and Tyr82) exhibit strong signal attenuation of signals after inactivation whereas residues indicated in dark red undergo structural rearrangements after inactivation as shown in Fig. 2A. Mutation of Gly377 equivalent to Chim Gly58 (orange) strongly affects the details of C-type inactivation in Kv1.3. Residues WT Ile60, WT Ser61, and WT Asp80 undergo chemical shift changes in KcsA gating mutants. According to MD simulations, TM1T residues 52 (WT) and 53 (Chim) are critically involved in protein–protein (KcsA) as well as protein–lipid (WT and Chim) interactions that stabilize the pore loop structure. (B) Side views shown for the case of the MlotiK1 (Left) and the Chim case (Right) with color coding as in A. For the sake of clarity, only a subset of residues is depicted in B.](/site-media/images/publications/2013/perozo-baldus-2013-figure5.jpg)

