A novel mechanism for fine-tuning open-state stability in a voltage-gated potassium channel
By Stephan A. Pless, Ana P. Niciforovic, Jason D. Galpin, John-Jose Nunez, Harley T. Kurata & Christopher A. Ahern.
Published in Nature Communications April 30, 2013;4:1784. PMID: 23653196. PMCID: PMC3644096. Link to publication page.
Core Facility: Membrane Protein Expression/Purification
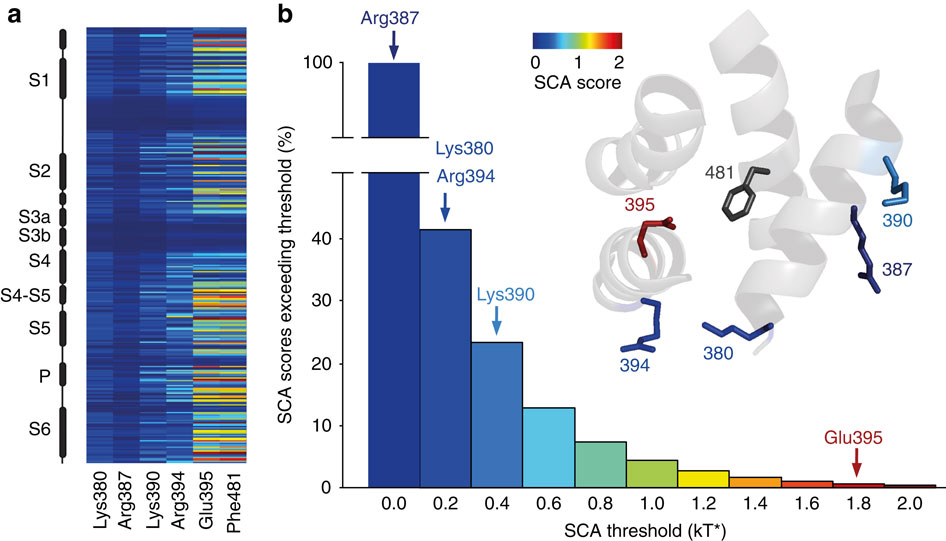
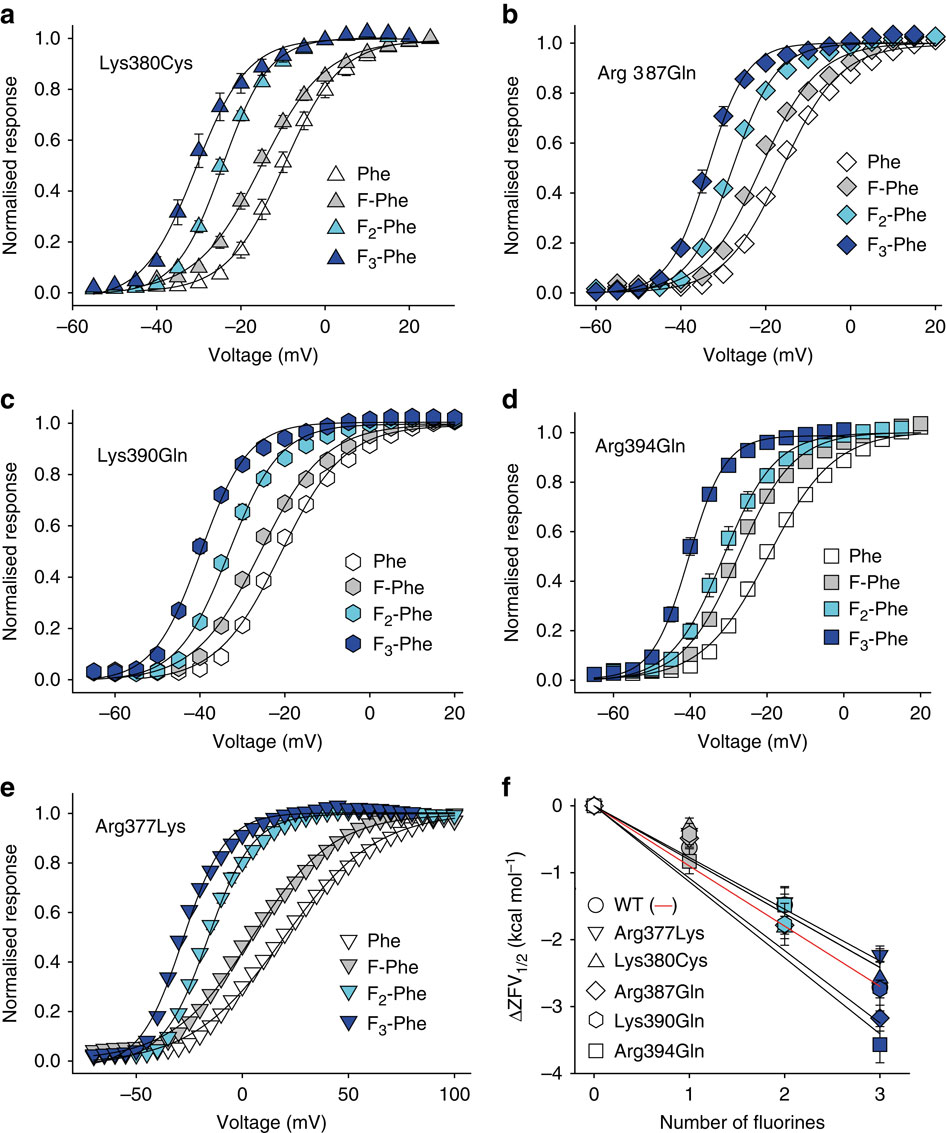
Figure 4: SCA analysis highlights the varying levels of co-evolution between Phe481 and nearby charged side chains. (a) SCA scores of Phe481 and all five charged side chains that are within 12 Å of Phe481 with the vertical axis representing all side chains considered for the SCA analysis (corresponding to positions 208 to 487 inShaker, with black boxes representing α-helical regions in the channel). Scale bar as in (b). (b) Bar diagram indicating the percentage of SCA scores exceeding the binned threshold indicated on the x axis for all pairings in the SCA analysis. The SCA scores of all five charged side chains with respect to Phe481 are highlighted with arrows (Arg387=0.13 kT*, Lys380=0.20 kT*, Arg394=0.21 kT*, Lys390=0.47 kT* and Glu395=1.93 kT*); Note the high co-evolution score for the Glu395-Phe481 coupling. Inset shows the scale of the heat map used in (a,b) and a model displaying all five charged side chains that are within 13 Å of Phe481. Note that the charged side chains are coloured by their SCA score values (Phe481 is shown in dark grey as all SCA scores are relative to Phe481 in this analysis).
Abstract
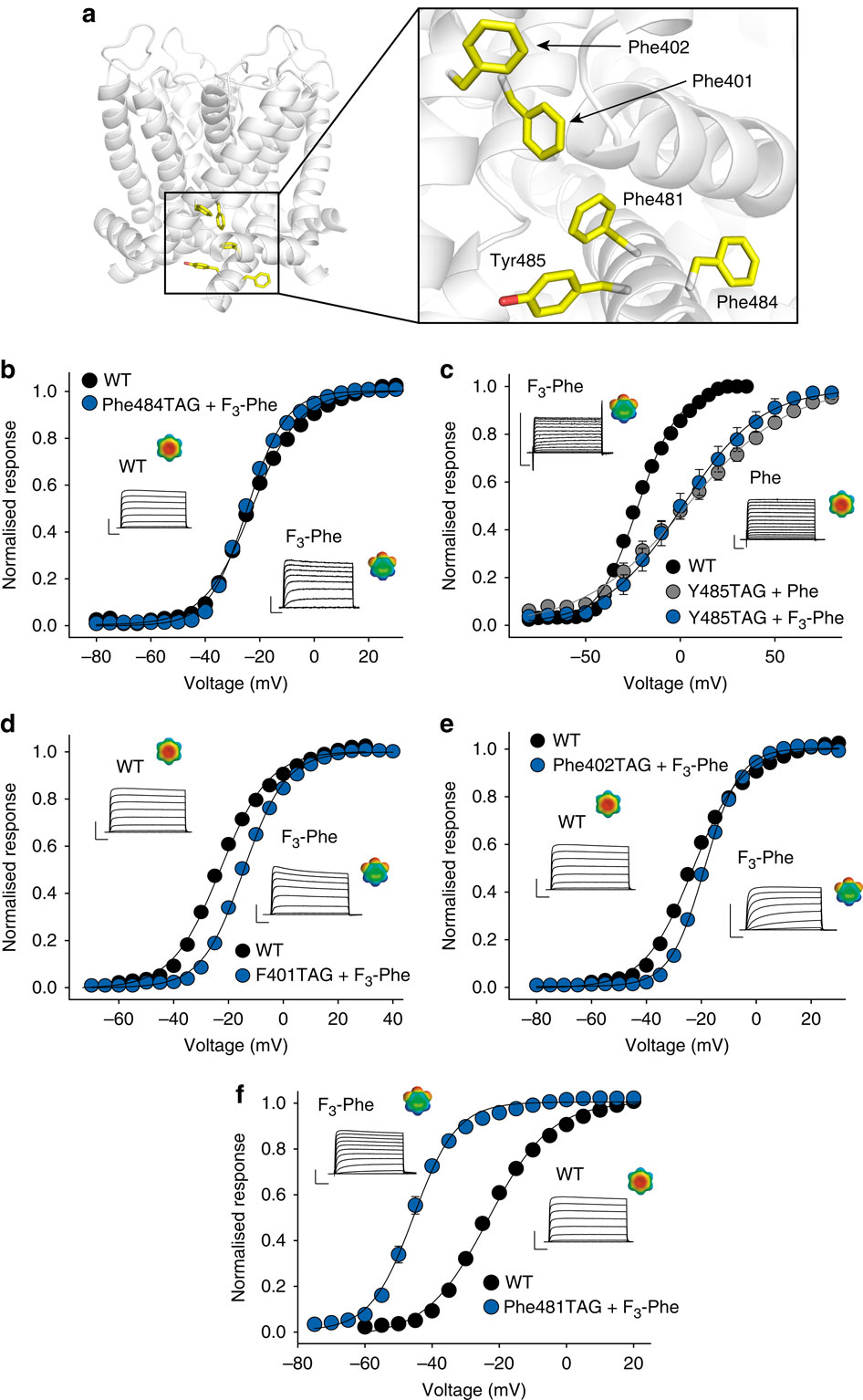
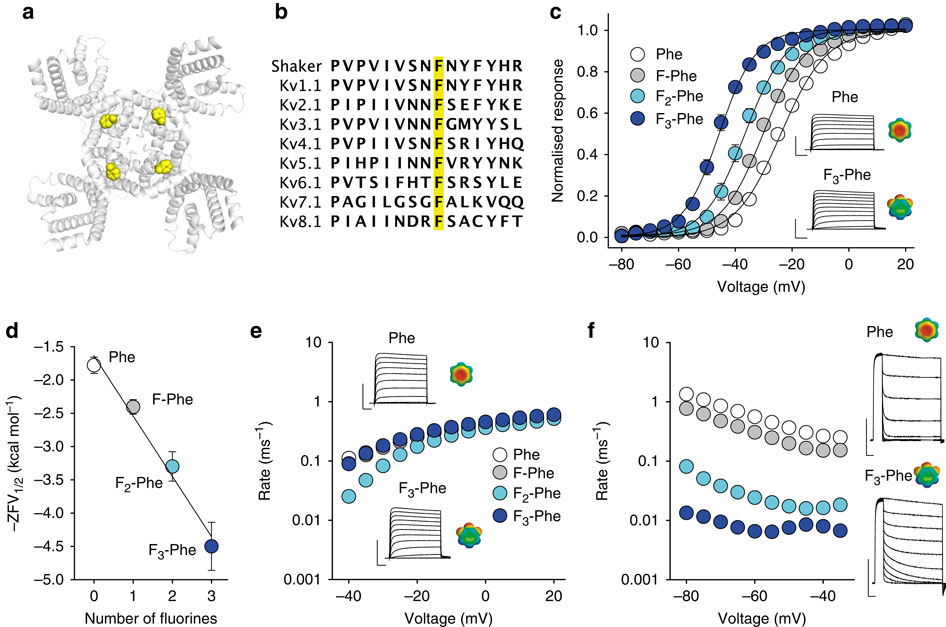
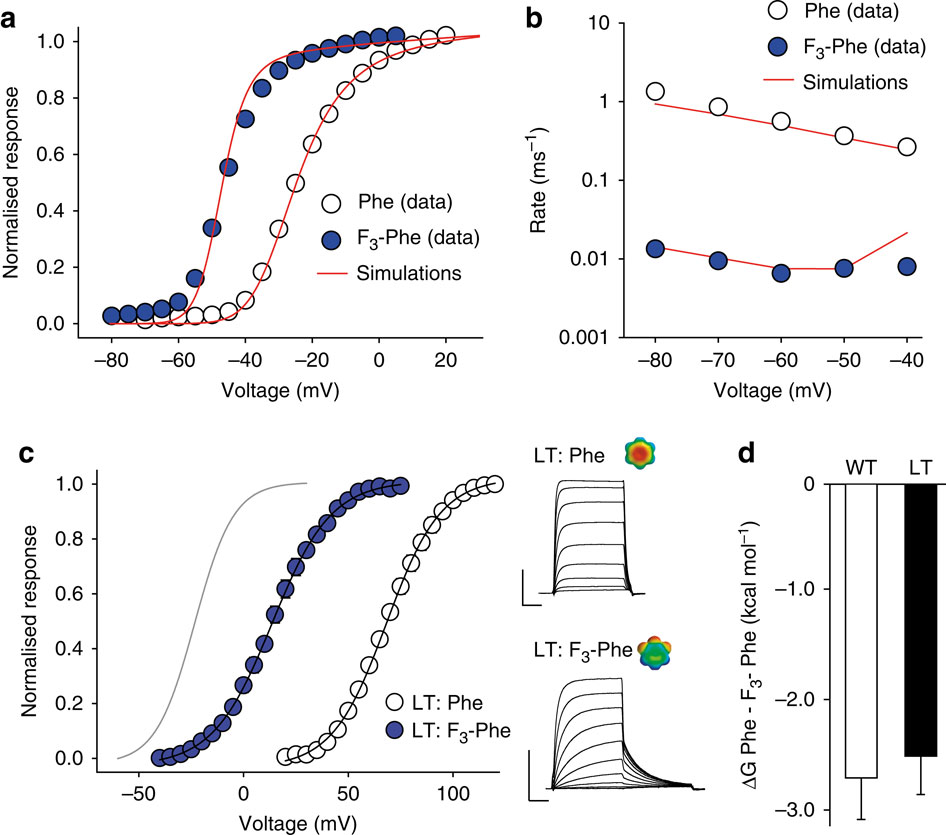
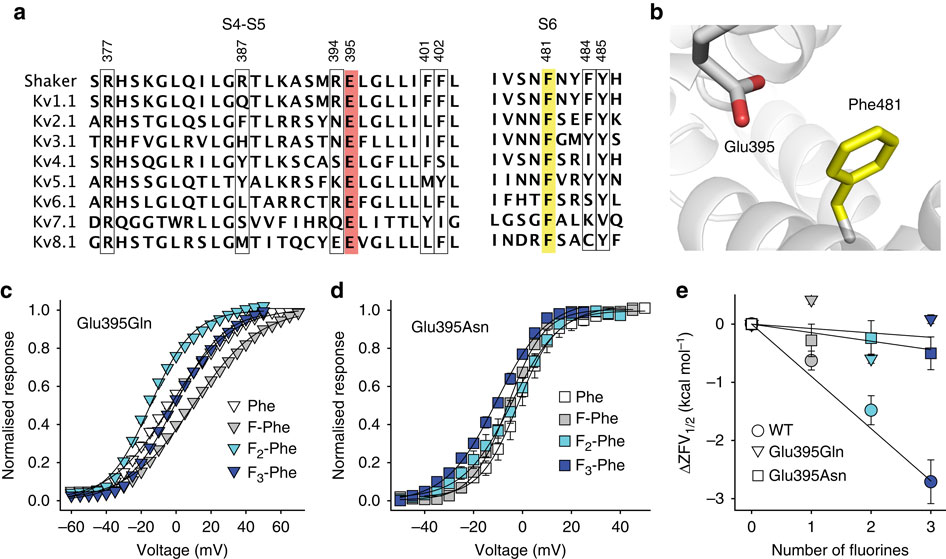
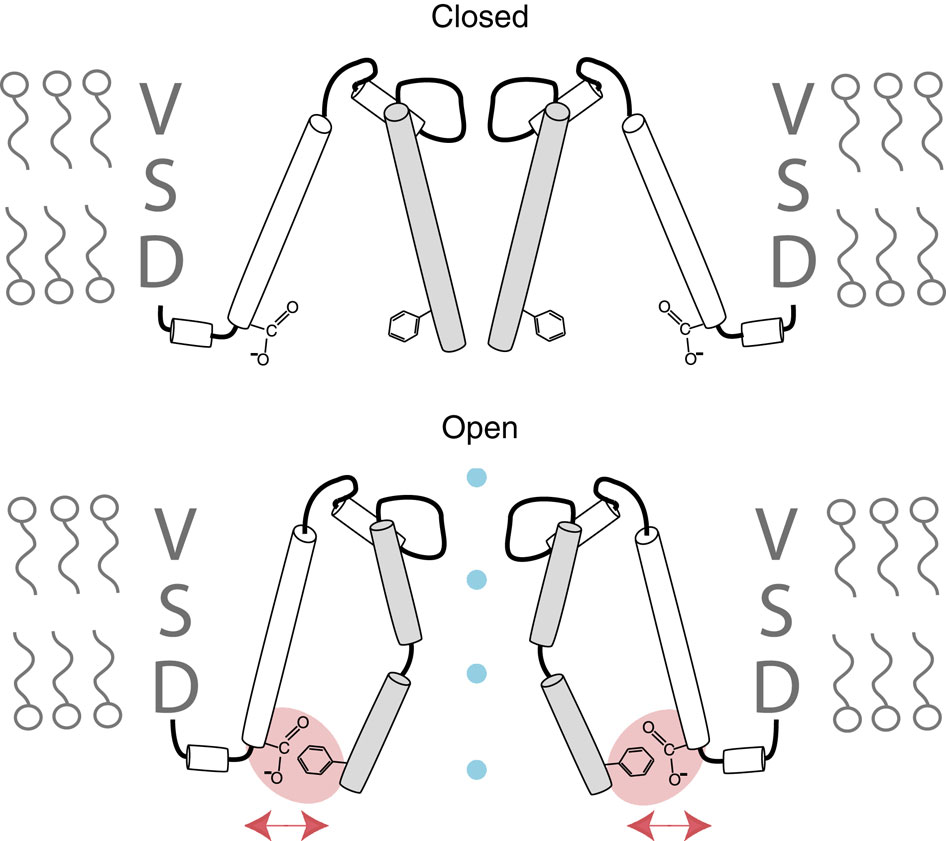
Voltage-gated potassium channels elicit membrane hyperpolarization through voltage-sensor domains that regulate the conductive status of the pore domain. To better understand the inherent basis for the open-closed equilibrium in these channels, we undertook an atomistic scan using synthetic fluorinated derivatives of aromatic residues previously implicated in the gating of Shaker potassium channels. Here we show that stepwise dispersion of the negative electrostatic surface potential of only one site, Phe481, stabilizes the channel open state. Furthermore, these data suggest that this apparent stabilization is the consequence of the amelioration of an inherently repulsive open-state interaction between the partial negative charge on the face of Phe481 and a highly co-evolved acidic side chain, Glu395, and this interaction is potentially modulated through the Tyr485 hydroxyl. We propose that the intrinsic open-state destabilization via aromatic repulsion represents a new mechanism by which ion channels, and likely other proteins, fine-tune conformational equilibria.