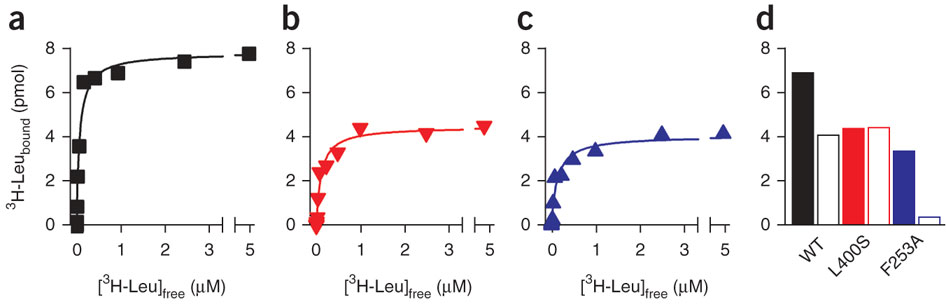
Experimental conditions can obscure the second high-affinity site in LeuT
By Matthias Quick, Lei Shi, Britta Zehnpfennig, Harel Weinstein, and Jonathan Javitch.
Published in Nature Structural & Molecular Biology 19(2): 207-11 on January 15, 2012.
PMID: 22245968. PMCID: PMC3272158. Link to Pubmed page.
Project: The Transport Cycle in Neurotransmitter Uptake Systems
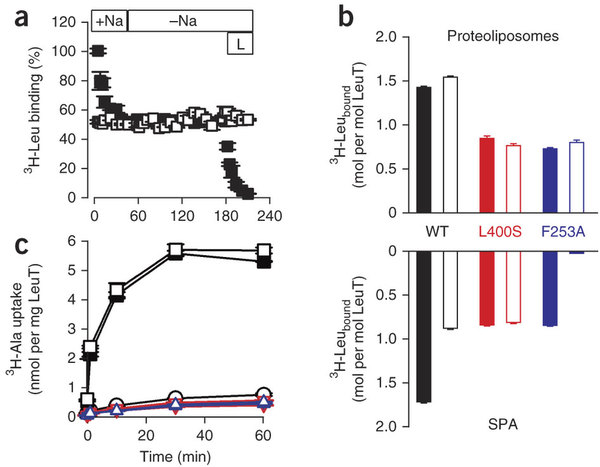
Figure 4: Intact S1 and S2 sites are required for Na+-coupled transport by LeuT. (a) Dissociation of 1 μM 3H-Leu from LeuT-WT by dilution into 50 mM Na+ (+Na) and into Na+ -free (−Na) media. Samples were preincubated in the presence of 0.1% DDM (▪) or 0.3% DDM (□) for ~500 h. Release of 3H-Leu trapped in the S1 site of LeuT assayed in 0.1% DDM was achieved by the addition of 2.5 μM leucine (L). (b) Binding of 500 nM 3H-Leu to 0.4 pmol LeuT-WT (black), LeuT-L400S (red) or LeuT-F253A (blue) in 0.1% DDM measured by SPA (lower panel) for 16 h using nonconcentrated (solid bars) or previously concentrated (open bars) material. Binding of 500 nM 3H-Leu to 0.4 pmol of protein was also assayed after reconstitution of previously concentrated or nonconcentrated LeuT-WT, LeuT-L400S or LeuT-F253A into proteoliposomes (upper panel). Equilibrium binding in proteoliposomes was carried out for 4 h in the presence of 25 μg gramicidin ml−1 (5-min pretreatment) to dissipate the Na+ electrochemical gradient, followed by capture of LeuT-containing proteoliposomes onto 0.22-μm nitrocellulose filters and subsequent scintillation counting. (c) Time course of Na+ -coupled uptake of 1 μM 3H-Ala in proteoliposomes reconstituted with LeuT-WT, LeuT- L400S or LeuT-F253A from nonconcentrated (solid black square, red inverted triangle and blue triangle, respectively) or concentrated (open black square, red inverted triangle and blue triangle, respectively) material, or in control liposomes (open black circle). Error bars in all panels are the s.e.m. of triplicate determinations from representative experiments that were repeated two or more times.
Abstract
Neurotransmitter:Na+ symporters (NSSs), the targets of antidepressants and psychostimulants, recapture neurotransmitters from the synapse in a Na+-dependent symport mechanism. The crystal structure of the NSS homolog LeuT from Aquifex aeolicus revealed one leucine substrate in an occluded, centrally located (S1) binding site next to two Na+ ions. Computational studies combined with binding and flux experiments identified a second substrate (S2) site and a molecular mechanism of Na+-substrate symport that depends upon the allosteric interaction of substrate molecules in the two high-affinity sites. Here we show that the S2 site, which has not yet been identified by crystallographic approaches, can be blocked during preparation of detergent-solubilized LeuT, thereby obscuring its crucial role in Na+-coupled symport. This finding points to the need for caution in selecting experimental environments in which the properties and mechanistic features of membrane proteins can be delineated.